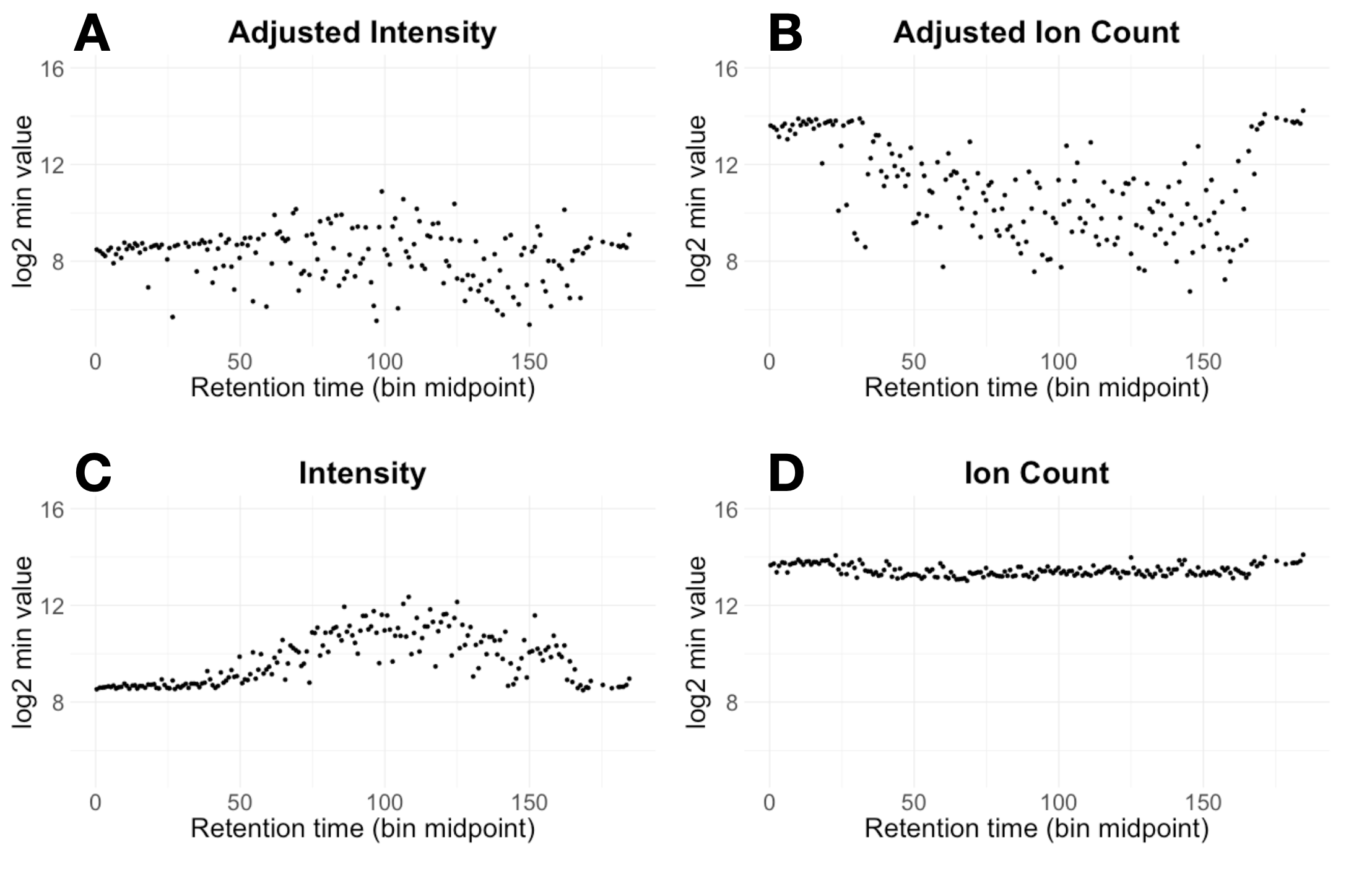
The Surprisingly Simple Fix: The LOQ is an Ion Count, Not an Intensity
The global LOQ has been hiding in plain sight. The key insight is that a mass spectrometer’s lower threshold is based on the minimum number of ions required to generate a signal. This threshold gets masked, however, when looking at intensities because intensities are adjusted by ion injection times. Varying ion injection times produce very different minimum intensities.
Here’s a simple analogy: catching 100 raindrops in 1 second tells you it's raining harder than catching 100 raindrops in 10 seconds. The same number of drops (ion counts), give represent very different intensities.
Some authors use signal-to-noise ratio as a surrogate for ion count, but we use defluxed intensities, which are simply intensity multiplied by ion injection time.
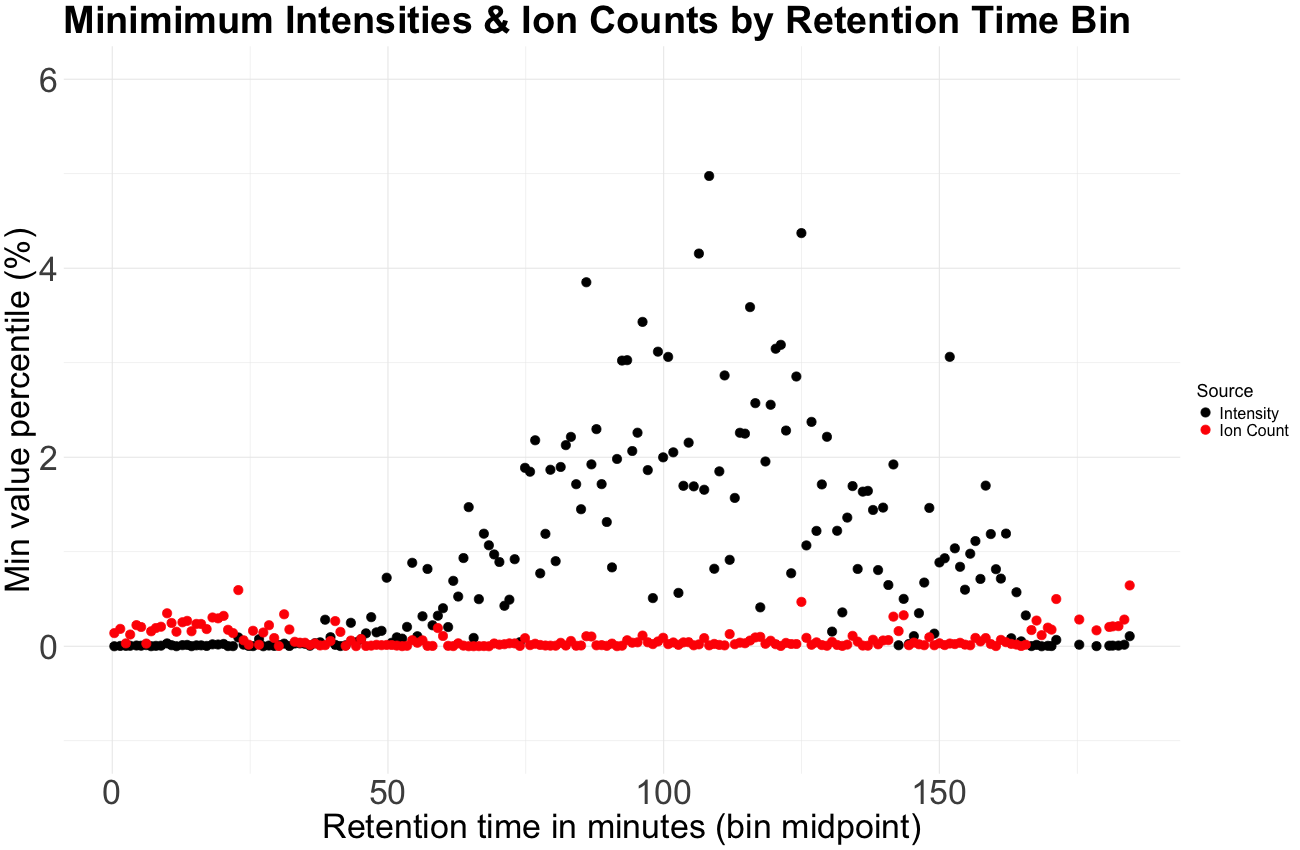
Figure 1: For an experiment with large fold-changes, we expect each sufficiently large sampling of peptides to contain a measurement from the minimum number of detectable reporter ions. We binned intensities and ion counts by retention time. For each bin, we plotted the minimum value as its percentile rank in the entire dataset. Ion counts show a consistent minimum across retention time while intensities do not. The minimum number of detectable ions is relatively consistent while the minimum intensity is not, due to varying ion injection times.